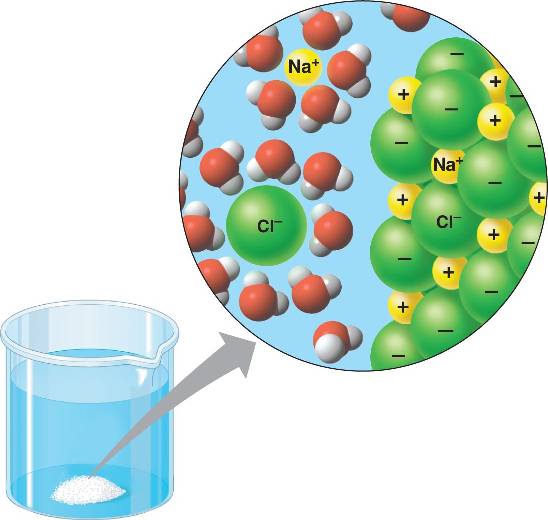
 Water is a versatile solvent for hydrophilic (polar and ionic)
substances
such as NaCl.
Water is a versatile solvent for hydrophilic (polar and ionic)
substances
such as NaCl.
Water molecules form hydration shells around ions, separating them from the crystal and dissolving the salt and forming a solution.
A solution with water as the solvent is called an aqueous solution.
Substances dissolved in solution are called solutes.